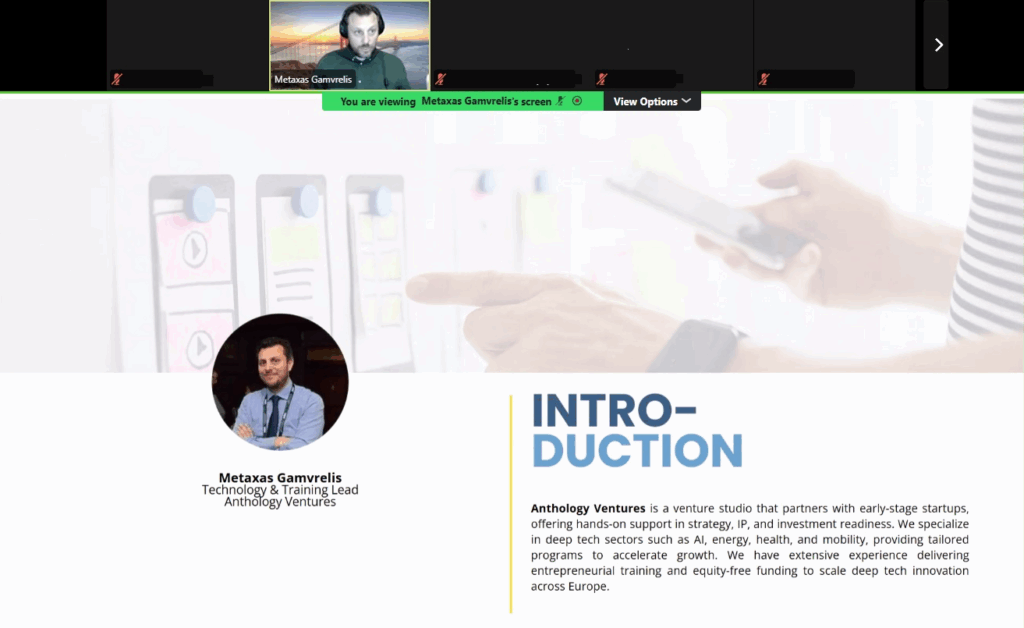
On June 12, 2025, the EVOLVE2CARE project successfully kicked off the first session of its Training Series for HealthTech Innovators and Researchers titled “From Users to Market – Faster Validation and Commercialisation for HealthTech Innovators and Researchers”, introducing participants to cutting-edge strategies for accelerating innovation through Large Language Models (LLMs) and No-Code platforms for rapid prototyping.
Led by Metaxas Gamvrelis, Technical & Training Lead at Anthology Ventures, the session “Accelerating Innovation — Leveraging LLMs & No-Code for Rapid Prototyping” brought together over 15 healthcare innovators and researchers, and offered a practical and inspiring deep dive into how tools and No-Code platforms like ChatGPT, Landbot, Lovable, and Google Gemini can dramatically shorten the innovation lifecycle—from early ideation to real-world deployment.
Rethinking prototyping: From weeks to days
Metaxas Gamvrelis opened the session by contrasting the traditional approach to prototyping—often slow, costly, and resource-intensive—with today’s possibilities enabled by AI and automation. He introduced a streamlined 4-stage framework that demonstrated how LLMs and No-Code tools transform each step:
Stage 1: Idea to Prototype
AI-LLMs can accelerate product ideation and planning by:
- Brainstorming product features and user stories
- Drafting Product Requirement Documents (PRDs)
- Generating user personas & customer journey maps
- Summarizing market research
Stage 2: User testing
Feedback is fuel for innovation. With AI-powered support, innovators can:
- Set up user tests quickly and with minimal resources
- Gather diverse feedback from surveys, interviews, and chatbots
- Perform initial analysis of results to identify key insights fast
- Perform sentiment analysis and theme extraction
Stage 3: Iterate & Improve
Once initial feedback is collected, the real power of LLMs and No-Code tools emerges, enabling rapid iteration, continuous refinement, and smarter decision-making without starting from scratch.
- LLMs help extract insights from user feedback, generate improvement suggestions, and update designs/requirements in real time.
- Tools like Lovable and Figma support versioning and A/B testing, making iteration cycles faster, smarter, and more agile.
Stage 4: Deploy Rollout (MVP)
With minimal development time, MVPs can be launched using No-Code platforms such as Glide, Replit, and Retool.
LLMs assist by:
- Generating product documentation
- Drafting marketing copy, landing pages, FAQs
- Creating onboarding flows (emails, chatbot scripts)
- Even code snippets for future integrations
Living Labs reimagined: More agile than ever
In the next part of the session, the training and technical expert explored how this tech revolution aligns perfectly with the Living Lab methodology, a cornerstone of EVOLVE2CARE project. By combining co-creation, iteration, and real-life user involvement with the agility of LLMs and No-Code:
- Prototypes can be created on-demand during co-design sprints
- Feedback can be gathered in real-time through smart forms and bots
- Iteration becomes virtually limitless within project timelines
“Living Labs are a huge gift,” he said, “because they help people check services before they’re fully developed. And now, they can do it faster, with less risk.”
To close the session, Metaxas Gamvrelis walked participants through real-time examples of how he uses ChatGPT in his own workflow. He emphasized the value of clear, well-structured prompts and shared one of his go-to techniques: “At the end of each prompt, I always ask: ‘Do you understand the task?’—it’s a small step that ensures accuracy.
What’s next?
This first session set the tone for EVOLVE2CARE’s Training Series—equipping HealthTech innovators and researchers with practical tools to accelerate early-stage development using LLMs and No-Code platforms. The second session, “Design Thinking in Action — A Living Lab Approach to Healthtech Innovation,” is scheduled for Thursday, 19 June 2025 at 15:00 CEST.
Join us as we dive into the power of design thinking in real-life healthcare contexts. Led by Ioannis Poultourtzidis, Coordinator of the ThessAHALL Living Lab, the session will explore how co-creation, inclusivity, and user-centered design lead to more relevant, ethical, and effective innovations.



 The Role of Living Labs in the Innovation Ecosystem | June 25, 2025 |15:00 CEST
The Role of Living Labs in the Innovation Ecosystem | June 25, 2025 |15:00 CEST Designing Tailored Living Lab Services for Innovators | July 9, 2025 | 15:00 CEST
Designing Tailored Living Lab Services for Innovators | July 9, 2025 | 15:00 CEST Navigating Legal, Ethical & Regulatory Frameworks | August 27, 2025 | 15:00 CEST
Navigating Legal, Ethical & Regulatory Frameworks | August 27, 2025 | 15:00 CEST Building Innovation Networks: Communication and Engagement | September 3, 2025 | 15:00 CEST
Building Innovation Networks: Communication and Engagement | September 3, 2025 | 15:00 CEST Certification & Standardization of Living Labs | September 10, 2025 | 15:00 CEST
Certification & Standardization of Living Labs | September 10, 2025 | 15:00 CEST Measuring Impact & Evaluating Success | September 24, 2025 | 15:00 CEST
Measuring Impact & Evaluating Success | September 24, 2025 | 15:00 CEST