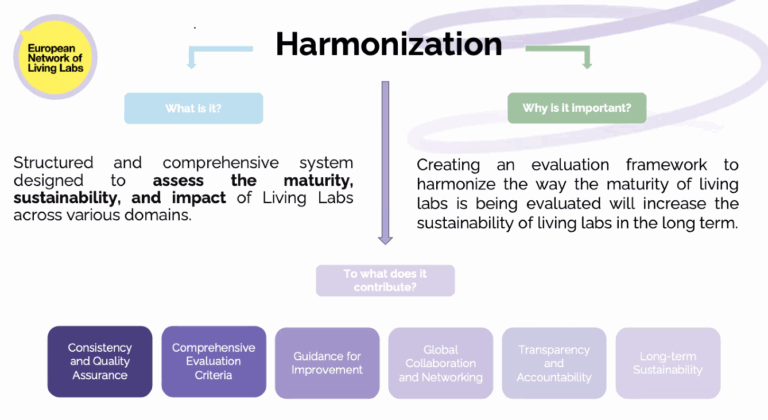
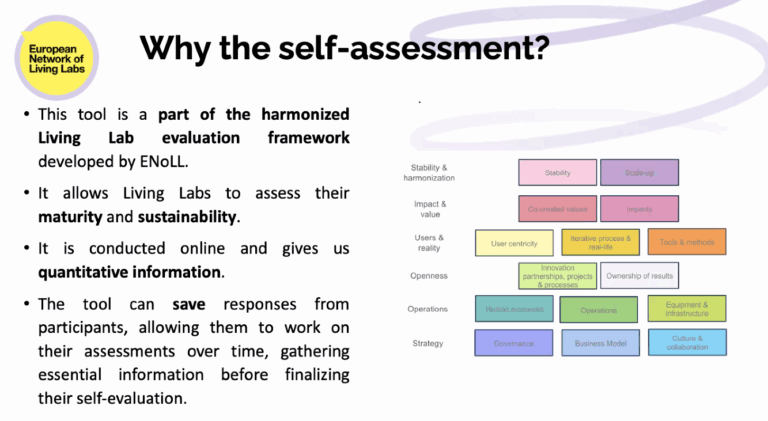
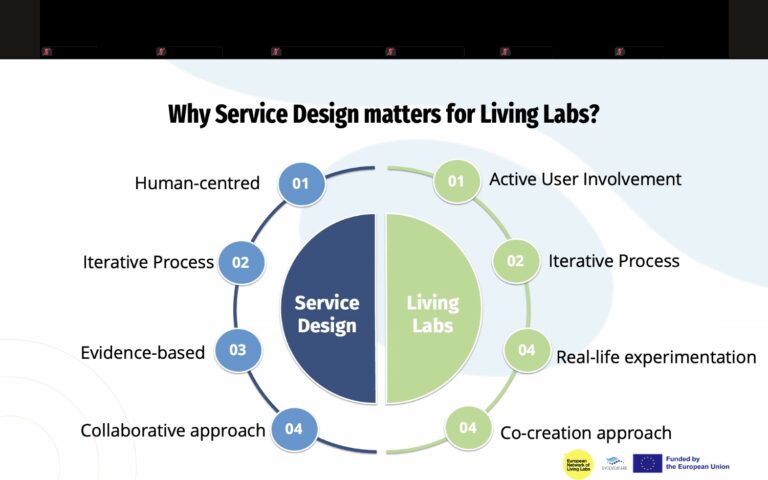
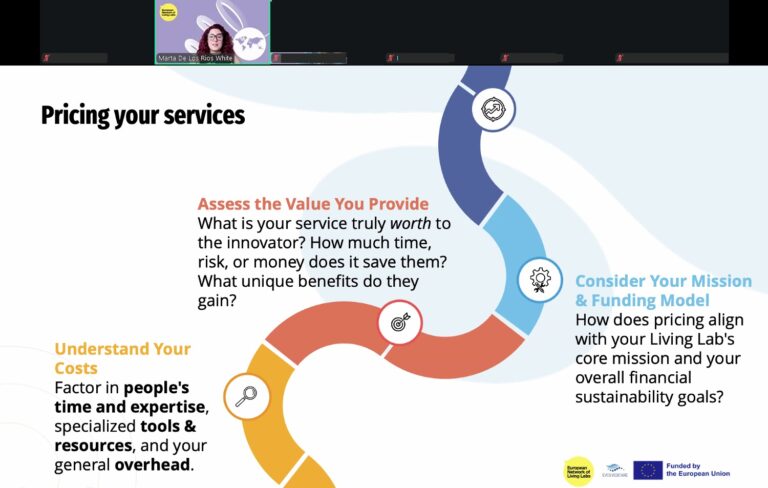
The fourth session of the EVOLVE2CARE Training Series for HealthTech Innovators and Researchers took place on 24 July 2025, bringing sharp focus to one of the most essential, and often overlooked, aspects of innovation: intellectual property (IP).
The session, titled “Unlocking IP Value – Protection, Collaboration & AI Innovations,” was led by Yannis Skoulikaris, Founder and Managing Director of PatentMind Netherlands BV, and former Director at the European Patent Office (EPO). PatentMind offers expert guidance based on deep knowledge of software patents, AI innovation, and international patent law.
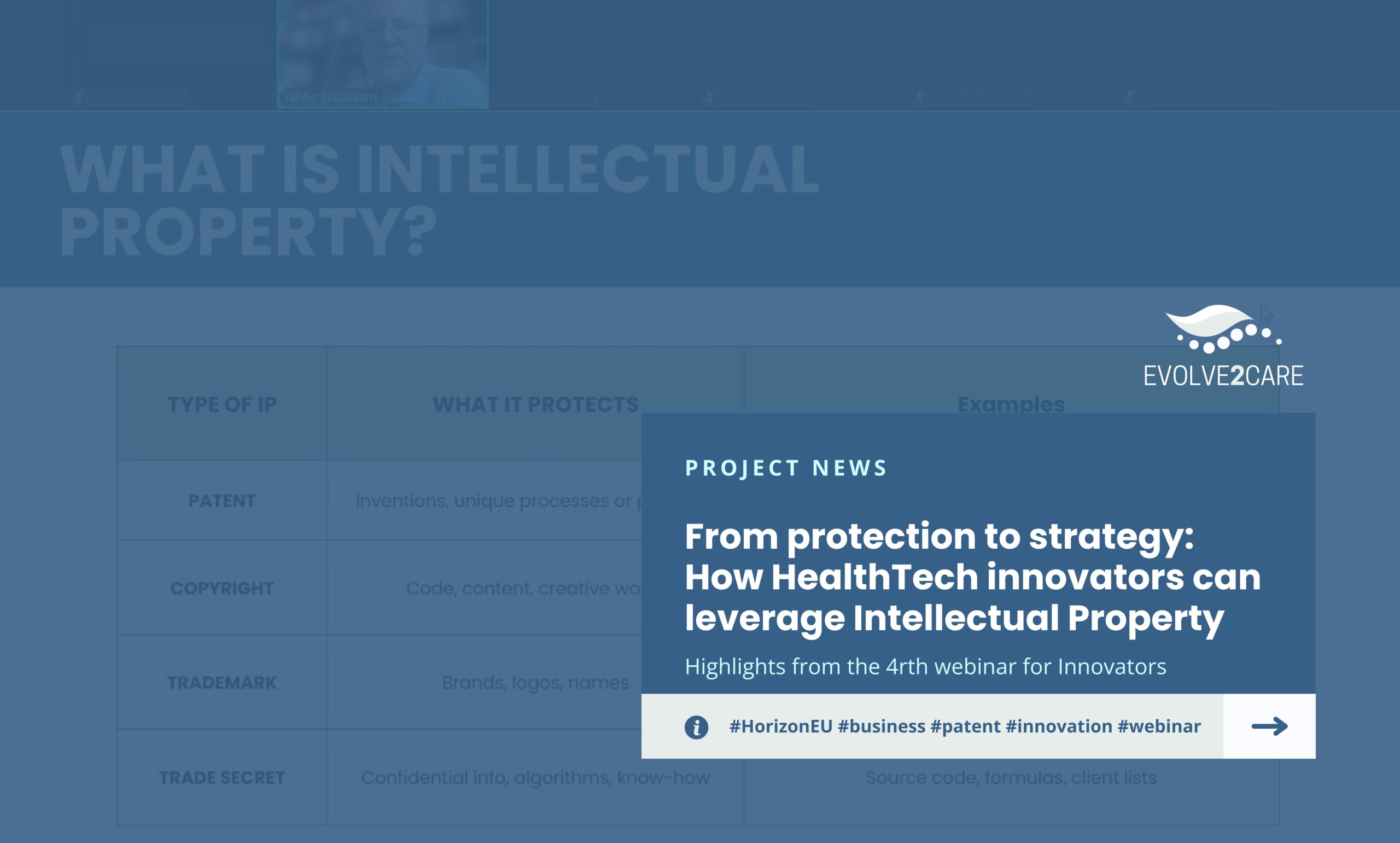
What is Intellectual Property, and what does it protect?
The session outlined the four types of IP and what they protect:
1. Patents – Protect technical inventions and unique processes or products
Patentable examples include:
- Algorithms, software methods, if embedded in a technical solution to a technical problem
2. Copyright – Protects code, content, and creative works
Patentable examples include:
- Source code
- Manuals
- Graphics
3. Trademarks – Protect brands, logos and names
Used for:
- Product names
- Company logos
4. Trade secrets – Protect confidential information, algorithms and know-how
Includes:
- Source more
- Formulas
- Client lists
The instructor highlighted that Intellectual Property is the secret weapon of innovators for protecting, managing, and unlocking value from their innovation.
Why does it matter?
- Competitive advantage
- Company Valuation & Investment
- Licensing Opportunities
- Legal defence & Risk mitigation
In the fast-evolving world of tech and AI, strong IP protection is crucial for staying ahead, attracting investors, and safeguarding innovation.
Patents in focus
Patents are one of the most powerful tools to protect and commercialize innovation—especially in HealthTech and AI-driven environments.
What can be patented?
To receive a patent, an idea must be novel and solve a real problem. The specific requirements vary slightly by region:
- In Europe, the invention must provide a technical solution to a technical problem.
- In the US, it must offer a useful solution to a practical problem.
Why do patents matter—particularly in tech and AI?
- They protect your innovations from being copied by competitors.
- They increase your company’s valuation and attractiveness to investors.
- They create new licensing and collaboration opportunities.
- Importantly, patent rights are granted to the applicant, not necessarily the inventor—making early filing and clear agreements essential.
How does the patenting process work?
The pathway from idea to protection follows a clear structure:
- Search: First, check if your invention is new.
- Application: File with the relevant patent office.
- Search/Examination: Patent office reviews your application in an interactive process, involving the applicant.
- Patent Granted
The power and peril of collaboration in IP
Collaboration is a cornerstone of innovation—but when it comes to Intellectual Property, shared ownership must be managed with clarity and care. Navigating shared IP ownership—whether with co-founders, collaborators, or licensees—requires balancing benefits and risks.
Why collaboration pays
Working with co-founders, collaborators, or licensees can accelerate development and expand your market reach. Benefits include:
- Shared expertise and resources, fostering deeper innovation
- Faster time-to-market through increased development capacity
- Broader visibility via partner distribution and co-marketing channels
The risks without IP agreements
- Ownership disputes – unclear title can derail projects
- Usage conflicts – unauthorized use or overlapping commercialization
- Enforcement issues or gridlock – difficult to license or defend jointly
Key takeaways
- IP is a critical asset: Proactively identify, protect, and manage your intellectual property
- Testing Innovation: Know which aspects of your work are eligible for protection
- Collaboration: Always set clear IP agreements at the start of any partnership
- AI’s unique IP challenge: Data, algorithms, and AI-generated works require tailored IP strategies
What’s next?
The next session in the EVOLVE2CARE Training Series for HealthTech Innovators and Researchers is titled “Fundraising & Pitching Strategies – An Investor’s Guide for Innovators.” It will feature Adriane Thrash, Managing Partner at Anthology Ventures, as the special speaker.
The session is scheduled to take place on 4 September 2025 at 15:00 CEST and will offer expert insights on how to craft compelling pitches and navigate the fundraising process with confidence. Designed for innovators looking to connect with investors and elevate their ventures, this event is a key opportunity to gain strategic guidance from an industry leader.
Stay tuned as we continue to bridge the gap between innovation and market with practical knowledge for real-world success.